Bioinorganic chemistry research
As a bioinorganic chemistry lab, our interests lie in the fascinating world of metal-containing enzymes. Our main project is centered on biological nitrogen fixation, the conversion of dinitrogen gas (N2) into ammonia (NH3). We are studying nitrogenase, the bacterial enzyme that reduces N2 into ammonia, and are also investigating regulatory proteins that influence nitrogenase activity and expression. In a previous project, we investigated heme uptake in Mycobacterium tuberculosis, the bacterium the causes the disease tuberculosis.
Nitrogenase
Nitrogen is abundant on earth, however, most of it is inaccessible to life since it is present as the inert gas N2, the most common gas in the atmosphere. Nitrogen fixation makes nitrogen bioavailable by converting N2 into NH3, which can be acquired by plants. Nitrogen fixation is especially critical in the context of agriculture since fixed nitrogen is essential for crop growth.
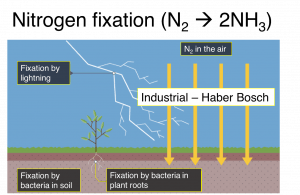
Overview of the different pathways of nitrogen entry into the biosphere.
Globally, there are two processes that account for the majority of fixed nitrogen. On the one hand, there is the industrial Haber-Bosch process in which N2 is converted to NH3 under large amounts of pressure and heat.
N2 + 3 H2 + 200 atm pressure + 400°C → 2 NH3
While the Haber-Bosch process has been indispensable in providing fertilizer for modern agriculture, it consumes vast amounts of energy and is not sustainable.
On the other hand, there is biological nitrogen fixation by nitrogenase. Unlike the Haber-Bosch process, nitrogenase uses renewable biochemical energy in the form of ATP to break the stable N2 triple bond to generate NH3.
N2 + 16 ATP + 16 e¯ + 8 H+ → 2 NH3 + H2 + 16 ADP + 16 Pi
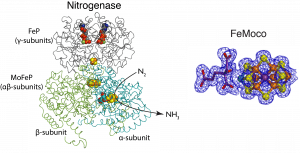
Nitrogenase is a unique enzymes from many points of view. Reduction of N2 to NH3 occurs via a unique metal cofactor in the active site of nitrogenase, called the Molybdenum-Iron cluster (FeMoco). In addition, nitrogenase contains a second unique cofactor, the P-cluster, and features a complex turnover mechanism that enables it to couple ATP hydrolysis to N2 reduction.
Nitrogenase crystal structure (left) and FeMoco active site (right) with electron density map shown in blue. Nitrogenase consists of two component proteins. The catalytic MoFeP and its reductase, FeP.
Nitrogenase regulation
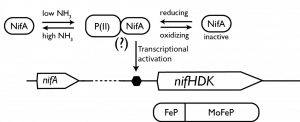
Nitrogenase is highly regulated. NH3 biosynthesis consumes large amounts of ATP and nitrogenase’s metal clusters are inactivated by common environmental gases such as O2. Therefore, diazotrophic bacteria have evolved mechanisms to regulate when nitrogenase is expressed. These regulatory processes are fascinating as they feature complex gas and redox sensing proteins that must work in consort to ensure nitrogenase is expressed at the right time.
Regulation of nitrogenase in alpha-proteobacteria by the central transcriptional regulator NifA that senses redox and cellular fixed nitrogen levels.
Current research
Nitrogenase mechanism
We are investigating the mechanism of nitrogenase catalysis in agriculturally relevant bacteria. We are particularly interested in understanding how different protein components in nitrogenase (the MoFeP and FeP) interact.
Nitrogenase regulation
We are characterizing the structure and redox sensing mechanism in alpha-proteobacterial NifA. NifA contains several metal clusters that play a role in redox sensing. The goal is this project is to learn how changes the clusters’ redox state are transduced to promote nitrogenase expression by NifA.
Protection of nitrogenase from inhibition by carbon monoxide
Nitrogenase is inhibited by the naturally occurring biological gas carbon monoxide. In many diazotrophs, a small protein called CowN prevents inhibition of nitrogenase. Our group discovered that CowN and nitrogenase interact and that CowN lowers carbon monoxides potency as an inhibitor. Currently, we are investigating the mechanism by which CowN exerts its protective effect.
Long-term objectives
Long-term, our objective is to use our understanding of nitrogenase and its regulation to enhance bacterial nitrogen fixation through biochemical engineering. We expect that by increasing diazotrophic NH3 output, we will lower the need for Haber-Bosch derived NH3 and decrease the environmental burden of agriculture on nature.
—
For information on our past bacterial heme uptake project, please click here.