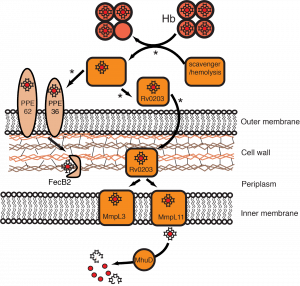
Bacteria, like all organisms, require iron (Fe) for growth. The iron-containing heme cofactor is abundant in humans and therefore represents a vast source of Fe for bacteria.
We are investigating how pathogenic Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, acquires heme from its human host. Tuberculosis represents a dangerous health threat and is one of the top-ten causes of death globally. The emergence of highly drug resistant strains of Mtb has increased the urgency to discover novel anti-Mtb drugs.
We have identified several proteins that are likely involved in heme uptake in Mtb. These proteins each bind heme and transfer heme between each other.
Heme uptake in M. tuberculosis. Rv0203, MmpL3 and MmpL11 bind heme, and Rv0203 transfers heme to both transmembrane transporters. Arrows marked by an asterisk indicate proposed steps that are under investigation. The PPE and FecB2 heme uptake pathway represents a second putative heme acquisition mechanism in Mtb.
Current research
Although we know Mtb can acquire heme, important aspects of its heme uptake pathway remain to be elucidated. It is not known if the extracellular heme binding protein Rv0203 directly acquires heme from host heme proteins or if a yet to be identified factor plays a role in heme scavenging. Moreover, we are investigating how heme is transported across the Mtb outer membrane and cell wall. Long term, we plan on using our knowledge of Mtb heme uptake to help guide the design of anti-tuberculosis drugs that inhibit Mtb heme uptake.
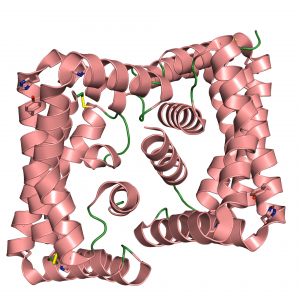
Crystal structure of the Mtb heme transport protein Rv0203.