Research projects in the LaRue Catalysis Lab largely focuses on using light (spectroscopy) to understand and control molecular interactions during chemical reactions. Individual projects invlove: using plasmonic nanopartcles to photoexcite chemical reactions (Core-Shell Plasmonic Photochemistry); understanding the fundamental processes that occur during chemical encounters on metal catalysts (Chemical Dynamics on Metallic Surfaces); and investigating the role calmodulin, a calcium sensing protein, plays in HIV viral replication (Interaction of calmodulin with the HIV-1 matrix protein).
Core-Shell Plasmonic Photochemistry
Catalysts are the hidden workhorses of chemistry in society: widely used in industry, they help reduce energy consumption, minimize pollution, synthesize chemicals, and produce food, to name a few. They accomplish all of these by lowering the activation energy barriers for desired reaction pathways, resulting in more efficient chemical reactions. Many catalysts used in industry today involve gas-phase reactants reacting on solid surfaces (heterogeneous catalysis). Despite their importance, these catalysts are often too inefficient to meet the global challenges we currently face, such as climate change.
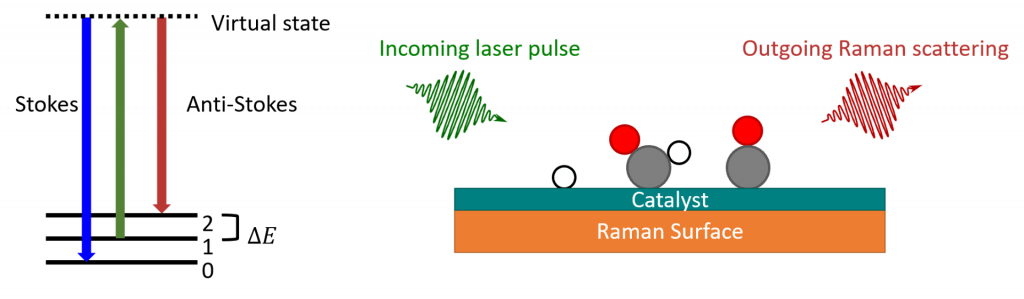
Plasmon-enhanced chemistry is a promising field for developing efficient and selective green photocatalysts, making them ideal candidates for clean solar-to-fuel conversion. Our group focuses on how the shape, size, and chemical composition of core-shell bimetallic plasmonic nanoparticles can be selectively altered for activating specific chemical reactions, such as CO formation, CO oxidation, and CO hydrogenation. A wide range of techniques, including Raman spectroscopy, mass spectrometry, SEM, and x-ray spectroscopies are used to characterize the physical and chemical properties of the plasmonic catalysts.
Chemical Dynamics on Metallic Surfaces
Today, we still know very little about the fundamental processes that occur during reaction conditions on the surface of metal catalysts. For example, the catalytic converter in automobiles was developed to convert harmful exhaust gases, such as carbon monoxide (CO) and nitric oxides (NOx), into less harmful or inert gases, such as carbon dioxide (CO2), oxygen gas (O2), and nitrogen gas (N2). The catalytic converter was developed through empirical data, instead of a fundamental understanding of the chemical reactions involved. One reason why chemical reactions are poorly understood on metal catalysts is that during reaction conditions, numerous individual reactions can occur simultaneously on the surface of the catalyst, each at different stages along the reaction pathway.
Projects are focused on isolating specific steps of reaction pathways in order to understand the most fundamental processes that occur during important chemical reactions on well-defined catalytic surfaces. This can be accomplished by using an ultra-high vacuum (UHV) surface science chamber, where catalysts are studied under ideal conditions. Using a UHV chamber with standard surface science components, we study the chemistry of small carbon molecules, including carbon monoxide, carbon dioxide, and methanol. Through collaborations with research groups at Stanford University and Stockholm University, we utilize x-ray spectroscopies and ultrafast optical laser techniques to study electronic structure and time-resolved dynamics of chemical reactions.
Interaction of calmodulin with the HIV-1 matrix protein
Human immunodeficiency virus type-1 (HIV-1) has caused over 35 million deaths, since 1981, and is the main cause of acquired immune deficiency syndrome (AIDS), which has led to over 34 billion dollars, globally, being invested in HIV-1 research per year. HIV-1 hinders the immune system’s ability to fight infections by attacking T cells, which are a central component of the immune system. HIV-1 infects T cells and uses their components to rapidly replicate, and can make about 10 billion copies of itself per day. The Gag protein of HIV-1 is crucial for the virus’s replication, and utilizes its essential matrix protein (MA) component to target the plasma membrane so that budding and assembly of the virus can occur. Calmodulin (CaM) is a calcium sensor with over 100 targets in eukaryotic cells. It is composed of a N-terminal and a C-terminal domain, each of which can bind up to two calcium ions, however CaM is able to function in the absence of calcium. MA forms two alpha helices upon binding to CaM, as opposed to one, making it a unique CaM-binding target with interactions that are not fully understood. MA consists of a myristoyl group which assists in targeting the plasma membrane, and it has been suggested that when CaM binds to MA, the myristoyl group of MA becomes exposed and is then anchored to the membrane. Using fluorescence spectroscopy and circular dichroism, we are investigating the binding of CaM to MA as a potential target to interupt the HIV replication cycle.
Techniques
We emply a range of spectroscopic methods to study chemical systems, including fluorescence spectroscopy, Raman spectroscopy, x-ray spectroscopies, and ultrafast optical laser techniques.
Raman spectroscopy is a technique that uses the inelastic scattering of light to provide information about bond energies for the reactants, intermediates, and products involved during chemical reactions.
X-ray spectroscopies, such as x-ray absorption spectroscopy (XAS), x-ray emission spectroscopy (XES), and x-ray photoelectron spectroscopy (XAS), provide element and chemically sensitive information about the atoms involved during chemical reactions.
Ultrafast optical laser techniques use the intense pulses generated by femtosecond lasers to initaite and/or probe chemical processes on surfaces. For example, in pump-probe spectroscopy, a femtosecond laser pulse is used to pump (excite) a chemical reaction on a catalyst, while a probe laser pulse (optical or x-ray) provides spectroscopic snapshots of the chemical environment as the reaction proceeds down the reaction pathways. Piecing together these snapshots, we can obtain an understanding of the step-by-step molecular processes that occur during chemical reactions.